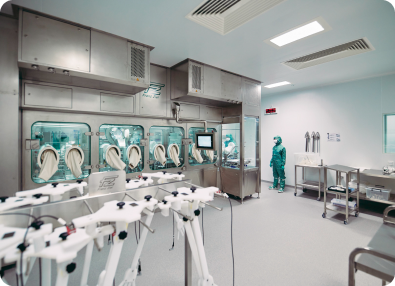
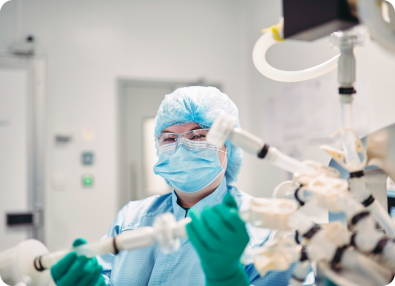
OXB has developed and optimised the analytics required for product commercialisation
Superior analytics allow us to understand both vector attributes and process performance throughout development and during routine quality control. The challenging analytical methods associated with complex products like lentiviral or AAV vector-based gene therapeutics requires specialist knowledge, techniques, and equipment.
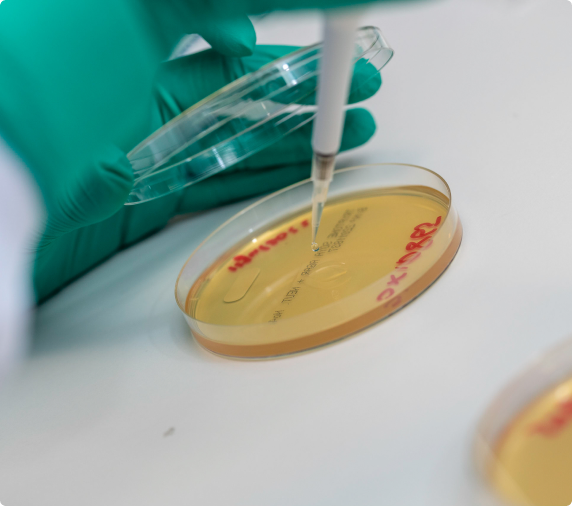
We offer an exceptionally comprehensive suite of in-house assays, ensuring full vector characterisation, quality control and stability testing, and preparing CMC modules for regulatory filings. We have an extensive clinical track record with our own and our partners’ products, and can advise our partners in the selection of appropriate assays.

We also develop custom-made assays for specific applications or types of product. OXB is one of the very few companies in the world that can offer in-house, CGMP, RCL (Replication-competent lentiviruses) testing from purpose-built category 3 labs. Our manufacturing sites are registered with the FDA and we hold manufacturing authorisations for clinical trial products and commercial products.

Our product assays
Platform assays
pH, Residual sodium butyrate, Endotoxin, Bioburden, Sterility, Mycoplasma, Micro BCA Total protein, HCP ELISA, Endonuclease, Picogreen Residual total DNA, 18S Residual host cell DNA, KanR Residual plasmid DNA, ABC-G Residual plasmid DNA, SV40 Residual host cell DNA

Lentiviral specific assays
Vector titre, FACS, RNA copy number, p24 ELISA, RCL, RCLCC
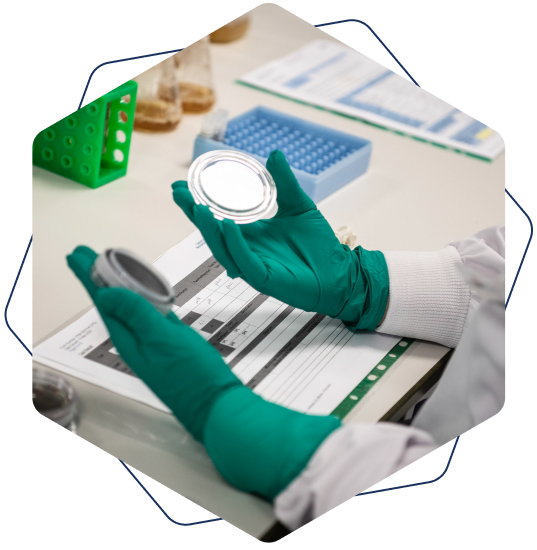
Product specific assays
Potentcy, FAC’s

Upcoming platform assays
Mass spectrometry, Next-Generation Sequencing, HPLC based vector quantification

AAV specific assays
Transgene expression, TCID50 for infectivity, In Vitro potency, Capsid titer by ELISA/GyroLab, Size Exclusion Chromatography (SEC) for aggregates, Analytical Ultracentrifugation (AUC) and CD-MS for empty/full/intermediate capsids, CE-SDS for capsid purity, Residual affinity ligand by ELISA and mass spec, Capsid identity by mass spec, Vector and Plasmid Characterization by NGS (PacBio)

See our other services
As a viral vector specialist with over 25 years experience in the cell and gene therapy industry, we have a thorough understanding of the challenges you face.