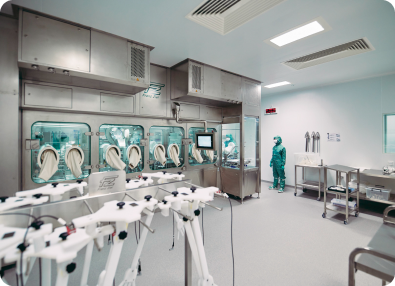
Lean on our process development expertise to help you reduce the time and expense involved in getting your next life-changing therapy to first clinical trial and beyond. We’ll collaborate with you to develop a strategy that streamlines your product development cycles and maximises yield – so you can increase your speed to clinic.

Serum-free production processes
Our serum and animal component free suspension processes use chemically defined media and supplements. This maximises volumetric productivity for a significant reduction in the vector cost per dose.

Giving you the commercial advantage
We’ve built quality by design into our process development service, to give you benefits including:
We’re all working towards the same end goal: to make cell and gene therapy universally available and affordable so that more patients can benefit from life saving interventions sooner.

See our other services
As a viral vector specialist with over 25 years experience in the cell and gene therapy industry, we have a thorough understanding of the challenges you face.