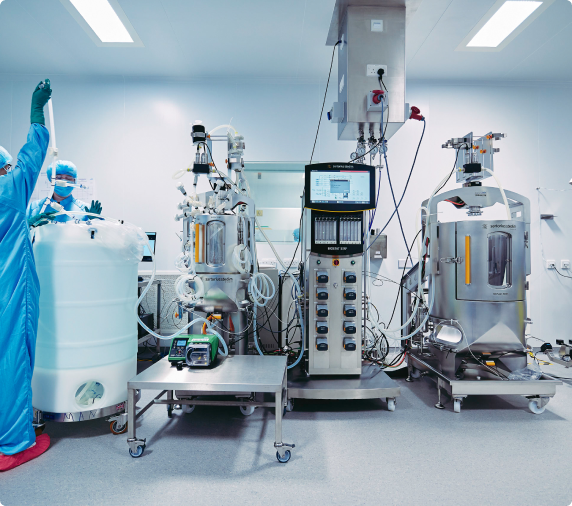
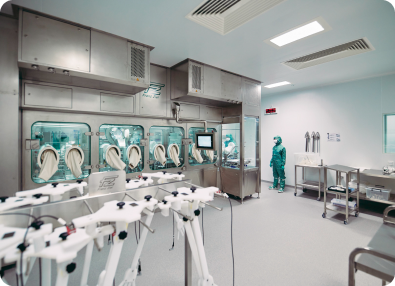
As a viral vector specialist with over 25 years of experience in the cell and gene therapy industry, we have a thorough understanding of the challenges you face.

We can reliably take you from laboratory concept through to clinical-grade manufacturing and large scale commercial production. Thanks to systems and processes that have been carefully refined over time, our end-to-end viral vector services come with the assurance that all of this will happen at pace, without ever compromising on safety.

Ways we can work together
We understand that your requirements are unique and never static, and that having a flexible partner to lean on will give you an invaluable advantage. We’re ready and equipped to join you at any or every stage of your development journey. You can depend on us to adapt to your needs and continually strive to exceed your expectations.

Tech transferred-in
Gold-standard execution of transferred-in technology and processes
Alongside our own LentiVector™ and inAAVate™ platform technologies, we have the facilities and expertise to fulfil almost any development need. You define the methods and processes, and we leverage our capabilities as appropriate.

Services Overview
A depth of experience in manufacturing and technical operations
Our experts have proven experience in collaborating with and delivering for clients. We offer experience supported by established, reputable policies and processes. Our end-to-end capabilities include:
See our other services
We partner with cell and gene therapy companies to provide innovative, end-to-end adeno-associated virus (AAV) process development and manufacturing services.