OXB has the technical and regulatory expertise to help you deliver the life-changing medicines of tomorrow.
Our commitment to medical innovation, proven experience of scaling up complex manufacturing processes and thorough understanding of product commercialisation give you everything you need to get your product to market.
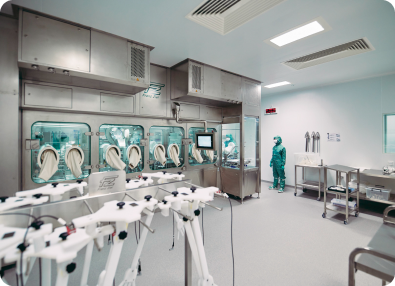
State-of-the-art facilities

Oxford, UK
Viral vector manufacturing in UK
Benefit from:
- 6 GMP manufacturing suites
- 200L and 1,000L bioreactors
- 2 fill finish suites
- 17,030m2 (183,300ft2) total size of facilities

Greater Boston, US
Viral vector manufacturing in the US
Benefit from:
- 4 GMP manufacturing suites
- 500L, 1,000L, and 2,000L bioreactors
- 1 fill finish suite
- 8,920m 2 (96,000ft2) size of facility

North Carolina, US
Viral vector manufacturing in Research Triangle Park, US
Benefit from:
- 2 GMP drug substance suites
- 1 GMP fill-finish suite
- ~11,240 m2 (~121,000 ft2) facility
- On-site QC labs and warehousing

Lyon, France
Viral vector manufacturing in France
Benefit from:
- 3 GMP manufacturing suites
- 200L bioreactors
- 1 fill finish suite
- 6,500m2 (70,000ft2) size of facility

Strasbourg, France
Viral vector manufacturing in France
Benefit from:
- 2 GMP manufacturing suites
- 200L bioreactors
- 1 fill finish suite
- 4,900m2 (52,700ft2) size of facility
Benefit from our deep scientific understanding
Working with OXB gives you access to capabilities that go far beyond conventional contract manufacturing services for viral vectors. Our commercial experience, scientific understanding, and development expertise complement our state of the art manufacturing facilities. Our teams are passionate about helping you get the results you want.

Benefit from our rigorous controls
When you use an OXB platform, you get a comprehensive, end-to-end CMC service. You can be sure our quality-focused GMP manufacturing processes will support your viral vector product through every step of development through to commercialisation, all supported by robust and compliant analytics.

See our other services
As a viral vector specialist with over 25 years’ experience in the cell and gene therapy industry, we have a thorough understanding of the challenges you face.