Comprehensive technology transfer for the pharmaceutical industry
As the cell and gene therapy sector matures, technology transfer has become a pivotal step for pharmaceutical companies striving to advance from early-stage research to clinical development and large-scale commercial manufacturing. Whether transitioning preclinical methodologies to clinical production, or scaling for market supply, the technology transfer process presents inherent complexities that require strategic execution.
As part of OXB’s CDMO services portfolio, we have developed a rigorous, phase-appropriate technology transfer framework tailored to meet the diverse needs of our clients. Our fit-for-purpose approach ensures the efficient transfer of processes and technical expertise while maintaining the highest quality standards and accelerated timelines. Our adaptable approach means clients are supported at every phase of development, whether they need early phase technical support, GMP manufacturing or commercial scale up.
OXB’s technology transfer process
Our technology transfer process is designed to be adaptable, meeting the unique requirements of each client at various stages of development. By leveraging a structured five-stage methodology, we ensure open communication, proactive risk mitigation, and a seamless transfer of manufacturing and analytical expertise and know-how.
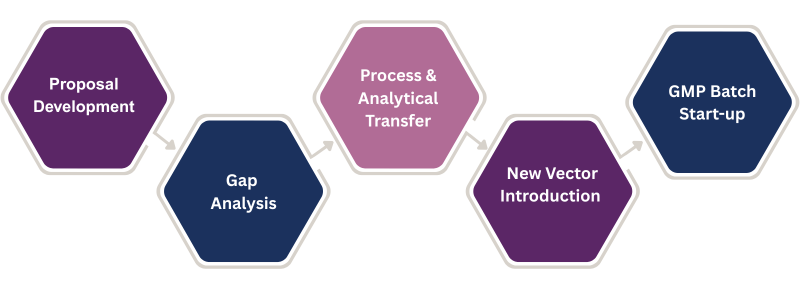
1. Proposal Development
Our dedicated proposals team collaborates closely with our Process Development (PD) and Manufacturing Science and Technology (MSAT) teams to create a comprehensive, tailored proposal that aligns with client-specific requirements.
2. Gap Analysis
A thorough process, analytical, and facility fit assessment is conducted, evaluating equipment compatibility, consumables, process flows, and scientific expertise. Our experienced teams identify potential risks and develop targeted mitigation strategies to ensure a smooth transition of client processes to OXB.
3. Process and Analytical Transfer
The defined process and analytical methodologies are then established within OXB systems. The required analytical methodologies are assessed within our QC laboratories, ensuring robustness and reproducibility.
A detailed process description encompassing upstream, downstream and fill/finish operations is created. Identified new material specifications are generated, along with a comprehensive bill of materials. Depending on the scale of transfer, further development work may be undertaken before scale-up to GMP manufacture.
4. New Vector Introduction
The product is introduced to the OXB GMP quality management system. Quality control testing and release requirements are established, along with the associated sampling plan. Details of the final product labelling is confirmed with the client. Product specific training is carried out to ensure operational readiness and compliance with regulatory standards.
5. GMP Batch Start-Up
Batch manufacturing records, product labels and sampling plans are issued. Final checks are completed to confirm that all batch documentation is in place prior to batch initiation.
Why Partner with OXB?
OXB’s technology transfer service is designed to accelerate the commercialisation of transformative cell and gene therapies. Our structured, phase-appropriate approach ensures a seamless and efficient transfer of critical knowledge, technology, and processes, enabling rapid clinical and commercial success.
 Leverage our proven track record in commercialisation
Leverage our proven track record in commercialisation
With over 30 years of experience in viral vector development, OXB has successfully delivered more than 30 Investigational New Drug (IND) applications and two Market Authorization Applications (MAAs). Our facilities have been inspected and approved by leading regulatory agencies, including the FDA, MHRA, EMA, ANVISA, and PMDA. Clients partnering with OXB can trust in our extensive expertise to navigate the complexities of commercialization with confidence.
 Streamline the process with a tailored, phase-appropriate approach
Streamline the process with a tailored, phase-appropriate approach
Our technology transfer strategy is meticulously structured to align with each stage of development, eliminating unnecessary activities and optimizing efficiency. This tailored approach ensures streamlined execution, reducing time to market without compromising quality or compliance.
 Access our analytical know-how
Access our analytical know-how
A critical aspect of technology transfer is the ability to implement and interpret robust analytical methodologies. With a portfolio of over 40 in-house assays, our highly trained scientists possess the expertise to select the most appropriate analytical methods and identify meaningful deviations, ensuring process integrity and product quality.
Speak to the OXB team about your technology transfer needs
At OXB, we are committed to driving the success of our partners through innovative technology transfer solutions for the pharmaceutical industry. By leveraging our extensive experience, regulatory expertise, and analytical capabilities, we empower our clients to bring life-changing cell and gene therapies to market with speed and precision. Contact us today to explore how our technology transfer services can support the next stage of your program’s success.